FREQUENTLY ASKED QUESTIONS
For quick reference, we answer questions that our clients often ask us below. If your question is not one of these, please call 1-(800) 649-4625, email us at sales@advcleanroom.com, or fill out our contact form. We welcome your questions and strive to be helpful.
Partnering with ACM means leveraging decades of expertise in cleanroom cleaning and contamination control to achieve effortless compliance and optimal performance in your operations. Whether your industry focuses on viable or non-viable particles, ACM’s specialized services provide measurable benefits:
Viable Particle Control
Industries such as vaccine production, bio-medical devices, pharmaceuticals, cosmetics, and food manufacturing face strict federal mandates to control viable particles like bacteria, viruses, molds, and spores. Daily decontamination is not just essential for safety—it is required to ensure products are safe for human use.
Benefits:
- Compliance with FDA and GMP regulations.
- Improved product safety and reliability.
- Increased production yields, directly boosting profitability.
Non-Viable Particle Control
For industries focused on non-viable particles, such as semiconductor manufacturing or electronics production, maintaining a cleaner environment often leads to significant yield increases.
Case Example:
A 1% increase in semiconductor output can translate into $50 million in additional annual revenue for larger companies.
Benefits:
- Reduced particulate contamination for higher production efficiency.
- Enhanced product quality and consistency.
- Fewer defects, minimizing waste and operational costs.
Data Center and Computer Room Maintenance
Clean, dust-free environments are critical for computer rooms and data centers, where contamination can cause clogged circuitry, equipment downtime, and even fire risks.
Benefits:
- Dust-free plenums ensure efficient cooling and operational stability.
- Prevention of costly downtime and equipment repairs.
- Reduced fire hazards and extended equipment lifespan.
ACM’s Comprehensive Services
ACM’s certified technicians deliver tailored solutions to meet the unique needs of your facility:
- Routine Cleaning and Disinfecting: Daily, weekly, or monthly schedules to maintain pristine cleanroom conditions.
- Construction Cleaning: Specialized services for post-construction contamination removal.
- Super-Cleans: Intensive cleaning for facilities requiring deeper decontamination.
- Environmental Monitoring: Ongoing assessments to track and manage contamination risks.
- Certification and Testing: Ensuring compliance with ISO, GMP, and other regulatory standards.
- SOP Writing and Training: Development of Standard Operating Procedures tailored to your facility, including training for compliance with the latest regulations.
Why ACM?
ACM’s licensed and certified associates ensure that your cleanroom and controlled environments are maintained to the highest standards. By prioritizing safety, compliance, and performance, ACM helps your business:
- Achieve effortless compliance with up-to-date federal and industry regulations.
- Maximize production yields while minimizing downtime and employee complaints.
- Deliver consistent quality and operational excellence.
The Result: Guaranteed effortless compliance and a trusted partnership for long-term success.
While janitorial cleaning focuses on general cleanliness and upkeep, cGMP (current Good Manufacturing Practices) cleaning requires a much higher level of expertise, precision, and regulatory compliance. At ACM, we ensure our technicians are thoroughly trained and equipped to meet the stringent demands of cleanroom environments, especially in FDA-regulated facilities.
The Foundation: Training and Certification
The differences between cGMP and janitorial cleans begin with the training process.
-
ACM’s Proprietary Training Program:
Every cleanroom technician undergoes rigorous training that includes:- Microbiology fundamentals to understand and control contamination sources.
- Cleanroom behaviors to minimize contamination risks.
- Aseptic gowning procedures and protocols.
- Good documentation practices (GDP) for accurate and compliant record-keeping.
- Specialized cleaning techniques tailored to controlled environments.
-
Site-Specific Training:
After completing ACM’s core program, technicians receive 1 to 8 weeks of site-specific training, covering the unique protocols, layouts, and compliance needs of individual facilities. -
Janitorial Training:
In contrast, janitorial cleaners are trained primarily in general cleaning tasks for uncontrolled environments such as offices, breakrooms, and public spaces. These tasks, while important, lack the complexity and regulatory oversight required for cleanrooms.
Documentation Practices: Precision Meets Accountability
In FDA-regulated facilities, cGMP cleaning requires documentation practices that are as rigorous as those of research scientists or production operators. This level of accountability ensures:
- Compliance with FDA and other regulatory body standards.
- Traceability of cleaning activities, critical in identifying and addressing contamination events.
- Confidence in maintaining a controlled environment for product safety.
Janitorial cleans, on the other hand, rarely involve detailed documentation or require adherence to strict compliance standards.
Continuous Education: Staying Ahead
ACM’s cGMP technicians engage in ongoing education to keep their skills sharp and their knowledge up to date. Continuing education modules cover:
- Handling and resolving deviations and excursions.
- Managing bacterial hits and maintaining product safety.
- Advanced cleaning protocols and personal safety practices.
This focus on continuous learning ensures that ACM’s technicians are always prepared to meet evolving industry standards.
The Bottom Line: A Specialized Skillset
Building a successful cGMP cleaner requires significantly more effort, expertise, and training than developing a janitorial cleaner for uncontrolled spaces. Cleanroom cleaners must:
- Understand microbiology and contamination control principles.
- Follow strict protocols to prevent contamination.
- Maintain compliance with rigorous documentation and regulatory requirements.
Janitorial cleaners, while skilled in maintaining general cleanliness, are not trained to handle the complexities of controlled environments.
Why Choose ACM?
ACM’s cGMP technicians are highly trained professionals who understand the critical role cleanrooms play in product safety and quality. When you choose ACM, you’re choosing:
- A team equipped with unparalleled expertise in cleanroom cleaning.
- A commitment to exceeding regulatory standards.
- Guaranteed effortless compliance for your facility.
The difference is clear: cGMP cleaning is not just about cleanliness—it’s about safeguarding your products, your facility, and your reputation.
This is one of the most frequently asked questions in cleanroom maintenance. Many cleanrooms are designed to exceed their classification requirements as defined by ISO-14644-2, and some particle count systems provide misleading results by sampling air within 6 inches of the filter face, which doesn’t reflect the true integrity of the room.
While meeting ISO-14644-2 standards is important, visible and non-visible particles pose risks that go beyond certification counts. Understanding their behavior and impact is crucial for ensuring the cleanliness of your cleanroom and the safety of your products.
Certification Particle Counts: A Limited Snapshot
During certification, particle counts are taken at working height and often under at-rest conditions, meaning no personnel are in the room. In these scenarios, most of the air sampled is undisturbed. However:
- Visible Particles: While noticeable, they typically have less impact on air particle counts due to their size and weight, which keeps them stationary unless physically disturbed.
- Non-Visible Particles: These smaller particles are far more mobile and can combine through molecular forces, eventually becoming visible to the naked eye.
The Risk: Disturbance caused by human activity, temperature or humidity changes, geological settling, or even molecular activity can mobilize both visible and non-visible particles, increasing the risk of product contamination.
Why Cleaning Matters, Even in Certified Rooms
Even if a room meets ISO-14644-2 standards, cleaning plays a crucial role in reducing contamination risks and maintaining optimal performance:
- Top-Down Supercleans: A thorough cleaning from ceiling to floor can reduce particles by 3 to 10 times in rooms that were not previously professionally cleaned.
- Continuous Professional Cleaning: Regular cleaning by trained staff ensures that particles are consistently removed before they can affect products.
- Interstitial Cleans: Cleaning hard-to-reach spaces, such as between equipment or in less accessible areas, further reduces contamination risks.
The Real Question: Is the Effort Worth It?
If cleaning reduces particle counts and removes large surface particles that could otherwise migrate, is it worth the effort for the product you’re manufacturing? The answer is almost always yes:
- Product Safety and Quality: Lower particle counts result in fewer risks of contamination, improving product reliability and safety.
- Regulatory Compliance: Continuous cleaning supports compliance with not only ISO standards but also other industry-specific requirements.
- Operational Efficiency: Cleanrooms with lower particle counts experience fewer defects and higher yields, directly benefiting profitability.
Why Choose ACM?
ACM specializes in providing tailored cleaning solutions, including:
- Top-Down Supercleans: Thorough removal of both visible and non-visible particles to optimize cleanliness.
- Continuous Maintenance Programs: Professional staff trained to maintain cleanroom integrity over time.
- Reduction of Particle Migration Risks: By addressing both large and small particles, ACM ensures your cleanroom exceeds expectations.
Meeting ISO-14644-2 standards is a baseline. Going beyond those standards with professional cleaning ensures your cleanroom remains a safe, efficient, and contamination-free environment for your critical operations.
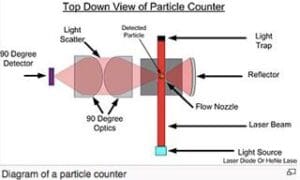
This is one of the most frequently asked questions in cleanroom operations—and for good reason. Cleanroom particle counts often rely on highly specialized systems, like the one illustrated in the diagram, to monitor air quality. These systems are designed to measure clean, undisturbed air and may not detect particles resting on surfaces, such as dust on ledges, until they are disturbed.
Understanding Particle Counting Systems
The diagram shows a typical particle counter setup, which measures particles suspended in the air using a combination of laser beams, optics, and detectors:
- Laser Beam and Light Source: Illuminates particles as they pass through the flow nozzle.
- 90-Degree Optics and Light Scatter Detector: Detects the light scattered by particles, allowing for size and concentration measurements.
- Flow Nozzle: Ensures controlled airflow through the detection system.
- Light Trap and Reflector: Focuses and contains the beam to improve measurement precision.
Most particle counters measure perpendicular to the airflow and near critical areas, such as within 6 inches of a filter face. While this approach is effective for monitoring airborne particles, it does not account for settled particles or the dynamics of particle disturbance.
Why Low Particle Counts Don’t Tell the Full Story
Particle counts are typically taken at working height and under controlled conditions (e.g., at rest or with minimal human activity). This means:
- Visible Dust on Surfaces: Particles on ledges or equipment do not contribute significantly to particle counts until they are disturbed.
- Non-Visible Particles: These particles, undetectable to the naked eye, can combine through molecular forces, eventually becoming visible and potentially problematic.
Disturbances caused by human movement, temperature and humidity fluctuations, or even geological shifts can mobilize these particles, increasing the risk of contamination despite low initial particle counts.
ACM’s Comprehensive Approach
ACM takes a broader approach to particle counting and contamination control, ensuring that surface and airborne particles are addressed:
- Monitoring Under Varying Conditions: We measure particle counts under different temperature, humidity, and activity levels to provide a more accurate assessment of contamination risks.
- Comprehensive Cleaning Protocols: By implementing top-down supercleans, interstitial cleaning, and continuous maintenance, we reduce particle counts by up to 10 times, even in certified cleanrooms.
- Surface Cleaning: Regular cleaning removes visible and non-visible particles from ledges and other surfaces to minimize mobilization risks.
The Key Takeaway
While particle counters provide valuable data on air quality, they don’t capture the full picture of potential contamination risks. Professional cleaning and ongoing particle monitoring are essential to maintaining the integrity of your cleanroom environment.
While cleanrooms are designed to control contamination, they are far from immune to particle accumulation. Regular cleaning is essential to maintain the integrity of the cleanroom environment and ensure product safety and compliance.
The Sources of Contamination
Even in a cleanroom, particles accumulate due to various factors:
-
Human Activity:
Humans are one of the largest contributors to contamination in cleanrooms. Skin flakes, hair, clothing fibers, and even the act of walking generate particles that can compromise cleanroom conditions. -
Manufacturing Processes:
Equipment operation, material handling, and production activities release particles into the environment. These particles often settle on surfaces or become airborne, posing contamination risks to products. -
Submicron Particles:
Not all particles are visible to the human eye or captured by filters.- Smaller, charged particles pass through filtration systems and combine with other charged particles, forming larger clusters.
- Over time, these clusters grow large enough to be seen, often appearing as dust or residue on cleanroom surfaces.
The Evidence: Dust Accumulation in Action
To demonstrate this, try placing a black fatigue mat on the cleanroom floor. Within hours, particles will begin to accumulate on the mat, even in the cleanest of cleanrooms. This visible dust is proof that particle generation is a continuous process, underscoring the need for regular cleaning to prevent contamination buildup.
Why Cleaning Matters
Regular cleaning ensures that contamination risks are minimized, even in environments designed to be clean:
- Improves Air Quality: By removing surface particles, fewer contaminants are disturbed and released into the air.
- Protects Product Integrity: Clean surfaces and air reduce the risk of contamination, ensuring products meet quality standards.
- Maintains Compliance: Adherence to cleaning schedules and protocols ensures ongoing compliance with regulatory and industry standards, such as ISO 14644 and GMP requirements.
The ACM Advantage
ACM provides tailored cleaning solutions to address the unique challenges of cleanroom environments:
- Continuous Maintenance: Daily, weekly, and monthly cleaning schedules to keep particle levels in check.
- Supercleans: Top-down cleaning to remove even the most stubborn particles.
- Environmental Monitoring: Ongoing assessments to identify and address contamination sources.
Even in the most advanced cleanrooms, contamination is inevitable without proper cleaning protocols. Partnering with ACM ensures your cleanroom remains compliant, efficient, and contamination-free.
ACM technicians are highly trained to clean your cleanroom and controlled environments with precision, targeting both visible and non-visible particles, as well as bacteria. Our customized cleaning programs are designed to address the unique contamination challenges your facility faces, ensuring the integrity of your cleanroom and the safety of your products.
Tailored Cleaning Programs
When developing a cleaning program, ACM considers two critical factors:
-
Particle Characteristics:
- The size of the particulate matter.
- The type of microorganism your product is most vulnerable to.
-
Adhesion Mechanisms:
- Particles adhere to surfaces through various forces, including:
- Electrostatic charges and ionic attraction.
- Humidity and gravity.
- Van der Waals molecular forces, which make smaller particles particularly difficult to dislodge.
- Adhesion and entrapment in uneven, oily, or adhesive-coated surfaces.
- Particles adhere to surfaces through various forces, including:
By understanding these factors, ACM technicians determine the most effective removal methods for your cleanroom environment.
Particle Removal Techniques by Size
-
Particles Larger Than 25 Microns:
These particles are often at rest and can typically be removed using high-efficiency vacuum cleaners. However, if they are trapped in uneven surfaces or contaminated by oil or adhesive, additional cleaning steps may be required to ensure complete removal. -
Particles Between 10 and 25 Microns:
Smaller particles require solutions that can effectively wet the particles, loosening their grip on surfaces for easy removal. -
Particles Smaller Than 10 Microns:
These are the most challenging to remove due to their size and molecular adhesion. ACM uses advanced techniques, including:- Physical Force: Precision wiping and brushing.
- Ultrasonic Waves: Vibrations that dislodge particles from surfaces.
- Solvents or Cleaning Solutions: Designed to dissolve or lift particles without damaging surfaces.
- Combination Methods: Utilizing multiple techniques for highly adherent or critical contamination.
Why Choose ACM for Particle Removal?
ACM technicians are trained to evaluate your facility’s unique decontamination requirements and apply the most effective cleaning methods. Our approach ensures:
- Complete Removal: From the largest visible particles to microscopic contaminants.
- Custom Solutions: Tailored cleaning programs that account for your specific risks and vulnerabilities.
- Ongoing Compliance: Cleanrooms maintained to meet or exceed ISO 14644 and GMP standards.
Whether your facility requires routine cleaning or advanced decontamination, ACM provides solutions that maximize cleanliness and minimize contamination risks.
Cleanroom cleaning programs are built on key components: proper staff training, specialized supplies, advanced equipment, precise methodologies, detailed cleaning schedules, and comprehensive documentation. Whether performing a Superclean, Interstitial Cleaning, or Continuous Cleaning, ACM ensures the highest standards are maintained to safeguard your cleanroom and products.
Superclean
A Superclean is an intensive, top-to-bottom cleaning designed to remove accumulated particles and contaminants that standard cleaning cannot address. It is often used during initial cleanroom setup, post-construction, or after an extended downtime period.
What It Includes:
- Top-Down Cleaning:
- Cleaning begins at the highest surfaces (e.g., ceilings, rafters, lighting grids) and moves downward to walls, equipment, and floors.
- Ensures that particles are dislodged and removed systematically, avoiding cross-contamination.
- Full Surface Coverage:
- Focuses on often-overlooked areas, such as T-bars, ductwork, plenum spaces, subfloors, and waffle decks.
- Includes detailed cleaning of all furniture, workstations, air vents, and pass-throughs.
- Specialized Supplies and Equipment:
- Use of ULPA-filtered detergents and vacuum cleaners filtered to 0.1 microns.
- Disinfectants are selected based on bio-contamination risks and tailored to the cleanroom’s purpose.
- Documentation:
- Every step of the cleaning process is recorded to ensure compliance with GMP and ISO standards.
Benefits:
- Removes deeply embedded particles and contaminants.
- Restores the cleanroom to optimal operating conditions.
- Essential for critical moments such as audits or product changes.
Interstitial Cleaning
Interstitial Cleaning focuses on hard-to-reach and often neglected spaces that can harbor particles and contaminants. It complements routine cleaning and helps maintain cleanroom integrity.
What It Includes:
- Targeted Cleaning of Hidden Areas:
- Rafters, ductwork, return air vents, subfloors, ceiling panels (both sides), and interstitial spaces between equipment.
- Specialized Tools:
- Precision cleaning tools designed to access narrow or obstructed areas.
- Preventive Contamination Control:
- Prevents particle migration from unseen areas into critical production zones.
Benefits:
- Reduces contamination risks from overlooked areas.
- Supports ongoing compliance and cleanroom performance.
Continuous Cleaning
Continuous Cleaning ensures that particle accumulation is consistently managed, maintaining a cleanroom’s operational integrity on a daily, weekly, or monthly schedule.
What It Includes:
- Frequent Cleaning of High-Touch and High-Use Surfaces:
- T-bars, lighting, walls, equipment surfaces, shelves, sinks, pass-throughs, airlocks, and floors are cleaned and disinfected according to pre-determined schedules.
- Rotational Disinfectants:
- Disinfectants are rotated to address bio-contamination risks effectively and prevent resistance.
- Meticulous Methodology:
- Cleaning begins at the cleanest areas (often furthest from the gowning room) and proceeds toward less clean zones, reducing cross-contamination risks.
- Customized Schedules:
- Frequencies are determined by the vulnerability of the product, personnel activity, and validation tests.
Benefits:
- Minimizes particle buildup and bio-contamination risks.
- Supports ongoing regulatory compliance and reduces downtime.
- Creates a consistent, contamination-controlled environment for production.
Comprehensive Training: The Foundation of Cleanroom Cleaning
ACM technicians undergo rigorous training that combines technical expertise with a deep understanding of why cleanrooms exist and how they function.
Training Includes:
- Fundamentals: Cleanroom design, particle behavior, and contamination risks.
- Technical Skills: Proper cleaning techniques, chemical safety, and cGMP compliance.
- Hands-On Application: Field training under qualified trainers ensures skills are practiced in real-world scenarios.
Why It Matters:
By understanding the “why” behind cleanroom protocols, technicians can make informed decisions and adapt to unique challenges in real time.
Key Considerations for Cleanroom Cleaning Programs
-
Supplies and Equipment:
- Cleanroom detergents and disinfectants are ULPA-filtered to 0.1 microns.
- Wipes, vacuums, and other tools are cleanroom-approved for compatibility and performance.
-
Methodology:
- Cleaning progresses from top to bottom and from cleanest to least clean zones.
- Frequencies and procedures are tailored to the product’s vulnerability and the cleanroom’s specific needs.
-
Class of the Room:
- While the class of the cleanroom affects gowning levels and air changes per hour, particle accumulation ratios remain consistent across classifications. Regular cleaning is essential regardless of class.
By combining Superclean, Interstitial Cleaning, and Continuous Cleaning into a comprehensive program, ACM ensures your cleanroom operates at peak performance, minimizing contamination risks and supporting your critical operations.
ACM ensures that all potential sources of contamination in your cleanroom are thoroughly cleaned on a schedule tailored to meet government regulations and customer-specific expectations. By addressing every critical surface, we help maintain the cleanliness and integrity of your cleanroom environment, ensuring your facility operates at peak performance.
Surfaces ACM Cleans:
-
Structural and Overhead Areas:
- Rafters, interstitial spaces, ductwork, plenum areas, and ceiling panels (both top and bottom).
- T-bars, lighting fixtures, and ionizing grids to ensure no particles settle in hard-to-reach areas.
-
Airflow Components:
- Return air vents and raised floors to maintain proper airflow and prevent particle migration.
-
Walls and Openings:
- Walls, windows, doors, pass-throughs, and airlocks are cleaned to prevent cross-contamination between zones.
-
Work Areas and Equipment:
- Workstations, equipment surfaces, cabinets, and shelves are sanitized to protect product integrity.
-
Fixtures and Furniture:
- Sinks, furniture, and other cleanroom fixtures are disinfected for consistent compliance.
-
Flooring:
- Floor mats, floors, sub-floors, and sub-fabs are meticulously cleaned to remove debris and eliminate contamination risks.
Compliance and Documentation
As part of ACM’s Guaranteed Effortless Compliance promise, we provide thorough documentation to ensure that all cleaning activities are properly filed for regulatory compliance. This includes:
- Detailed reports of cleaning schedules and procedures.
- Validation of all surfaces cleaned according to ISO, GMP, or other relevant standards.
- Evidence of compliance for audits or inspections.
By addressing every critical surface in your cleanroom, ACM minimizes contamination risks, ensures regulatory compliance, and supports your operational excellence.
Ensuring that your cleanroom is clean requires a combination of visual inspection, surface and airborne particle counts, and microbial environmental testing. These methods provide comprehensive insights into the cleanliness of your cleanroom and its readiness for production.
1. Visual Inspection
A simple yet effective way to assess cleanliness is by conducting a visual inspection:
- Angle Observation: Look at surfaces at approximately a 10% angle to identify particles that may not be visible at first glance.
- Surface Clarity: Check for visible dust, smudges, or residues on walls, floors, and equipment surfaces.
While visual inspection alone doesn’t detect microscopic particles, it is a critical first step in identifying problem areas.
2. Surface Particle Counts
Surface counters electronically measure particles down to the molecular level:
- High-Sensitivity Detection: These tools capture data on both visible and non-visible particles.
- Proactive Monitoring: Regular surface particle counts allow for early identification of potential contamination.
3. Fall-Out Counts
Fall-out counts involve collecting particles that settle over time using petri dishes or other substrates:
- Microscopic Analysis: Samples are examined under a microscope to detect small or otherwise invisible particles.
- Environmental Trends: This method is particularly effective for identifying long-term contamination trends in low-traffic areas.
4. Airborne Particle Counts
Airborne particle counters measure particle concentration in real-time:
- Dynamic Sampling: Counts are taken while the room is at rest and during operation to capture the full spectrum of particle activity.
- Accurate Measurements: Instruments can detect particles as small as 0.3 microns, ensuring detailed data on air quality.
5. Microbial Environmental Testing
This testing identifies viable particles, such as bacteria, yeasts, molds, and spores:
- Growth Media Analysis: Petri dishes are used to collect microbial fall-out, which is then incubated to determine contamination levels.
- Comprehensive Safety Assurance: Results help ensure that viable particle levels remain within acceptable limits for your operations.
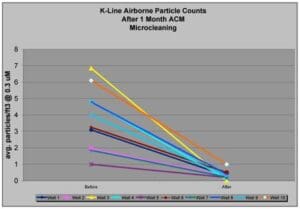
Proven Results: Particle Reduction with ACM Microcleaning
As shown in the provided chart, ACM’s microcleaning programs significantly reduce airborne particle counts, resulting in cleaner environments:
- Before Cleaning: Initial particle counts are higher due to accumulated contaminants.
- After Cleaning: Following ACM’s thorough cleaning, particle counts consistently decrease by 2 to 10 times, depending on the level of cleaning and room usage.
This reduction not only improves air quality but also ensures compliance with regulatory standards and enhances production yields.
Tailored Cleaning Programs for Your Needs
Whether your concerns focus on viable particles, non-viable particles, or yield improvements, ACM develops customized cleaning programs to keep your cleanroom operating at peak performance. With a focus on effortless compliance and measurable results, ACM ensures that your cleanroom meets and exceeds cleanliness standards every time.
ACM’s certified technicians are highly trained to clean controlled environments using our proprietary “We-Stake-Our-Reputation-On-It” cleaning methodology. This systematic approach ensures the thorough removal of particulate matter and minimizes cross-contamination, maintaining your cleanroom’s compliance and operational efficiency.
Our Cleaning Process
-
Top-Down Cleaning:
- Cleaning begins at the highest surfaces, such as ceiling panels, rafters, and lighting grids, ensuring that particles dislodged during cleaning are captured as they move downward.
- Each surface is carefully cleaned and documented to ensure no area is overlooked.
-
Clean-to-Less-Clean Flow:
- Cleaning starts at the cleanest end of the room, typically the production or operation area, and progresses toward the least clean zones, such as gowning rooms or entryways.
- This flow reduces the risk of cross-contamination and maintains cleanroom integrity throughout the process.
-
Meticulous Exit Strategy:
- As technicians clean their way out of the room, every step is carefully planned to ensure no recontamination occurs.
- This includes cleaning pass-throughs, doors, and airlocks as the final steps before exiting.
Key Features of ACM’s Cleaning Methodology
-
Comprehensive Surface Coverage:
- All critical cleanroom surfaces, including ceilings, walls, floors, equipment, and furniture, are addressed during the cleaning process.
- Specialized tools and cleaning solutions are selected based on surface type and contamination risk.
-
Maximum Particle Capture:
- ACM’s cleaning techniques are designed to capture the maximum amount of particulate matter, including both visible and non-visible particles.
- ULPA-filtered vacuum systems, cleanroom-grade detergents, and disinfectants are utilized to ensure thorough decontamination.
-
Minimal Cross-Contamination:
- By following a top-down, clean-to-less-clean methodology, ACM technicians prevent the spread of particles between zones.
- This method ensures that contamination risks are minimized at every stage of the cleaning process.
Why ACM?
Our technicians receive rigorous training in both technical cleaning skills and the science behind cleanroom contamination. This ensures that:
- Every technician understands why cleanroom protocols exist and how to make real-time decisions to maintain compliance.
- Your cleanroom is cleaned to meet or exceed ISO 14644, GMP, and other regulatory standards.
- Documentation is provided to support audits and ensure compliance at all levels.
The Result: Guaranteed Effortless Compliance
With ACM’s industry-leading cleaning methodology, you can trust that your controlled environment is maintained to the highest standards, protecting your products, processes, and reputation.
WE ARE SO GLAD YOU ASKED THIS QUESTION! Proper preparation before hydrogen peroxide fumigation is critical to ensure the efficacy of the sanitization process. Fumigation, also referred to as vaporization, is the dispersal of a sanitizing solution into the air to address surface contamination. However, fogging alone does not clean gross contamination.
Why Pre-Fumigation Cleaning Is Essential
Fumigation works by allowing vaporized hydrogen peroxide (H₂O₂) to contact microorganisms directly. If surfaces are covered by contaminants like dust, debris, papers, or moisture, the vapor cannot penetrate these barriers effectively, significantly reducing its sanitizing power.
- Gross Contamination Risks:
- Dust and debris can harbor microorganisms, allowing them to remain viable for extended periods.
- These contaminants act as a physical “umbrella,” shielding microorganisms from direct contact with the vapor.
Bottom Line: Fumigation without proper cleaning compromises the process and may leave harmful microorganisms intact.
Steps to Prepare the Room for Fumigation
To maximize the effectiveness of hydrogen peroxide fumigation, a Superclean must be performed beforehand to expose as much surface area as possible. Here’s how to prepare the space:
-
Remove Obstructions:
- Dispose of unnecessary items, papers, boxes, and trash.
- Empty drawers, cabinets, and other storage spaces.
-
Create Airflow Around Objects:
- Elevate items that cannot be removed to allow vapor access underneath and around them.
-
Address Equipment and Fixtures:
- Identify and prepare items like safety cabinets, fume hoods, laminar flow benches, incubators, refrigerators, and freezers.
- Ensure provisions are made so vapor reaches all surfaces of these fixtures.
What Is Hydrogen Peroxide Fumigation?
Hydrogen peroxide vapor (H₂O₂) is a highly effective surface sanitizer that uses free radicals to deactivate microorganisms, including:
- Bacteria
- Viruses
- Molds
- Fungi
How It Works:
- Kinetic Energy: Vaporized H₂O₂ generates superoxide and hydroxyl radicals that physically interact with and destroy microorganisms.
- Deactivation Process: Once bio-deactivation is complete, the vapor is catalytically converted into harmless water (H₂O) and oxygen (O₂), leaving no residue.
Key Points to Remember
-
Fogging Is NOT a Substitute for Cleaning:
- Dust and debris must be removed to allow full vapor penetration.
- Without pre-fumigation cleaning, sanitization will be incomplete.
-
Physical Characteristics Matter:
- Cleanroom layout, equipment placement, and airflow must be considered to ensure all surfaces are reached.
-
Thorough Cleaning Is Non-Negotiable:
- Surfaces must be free of gross contamination to ensure the fumigation process is effective.
- Even in doubt? Repeat: It is impossible to over-emphasize the importance of thorough cleaning prior to fumigation.
Why Choose ACM for Pre-Fumigation Cleaning?
ACM’s certified technicians are trained in meticulous cleaning techniques to ensure your cleanroom or controlled environment is fully prepared for fumigation:
- Top-Down Supercleans: Removes particles and contaminants from ceilings to floors.
- Detailed Equipment Preparation: Ensures complete access for vapor penetration.
- Guaranteed Compliance: All cleaning is documented and performed to meet regulatory and industry standards.
By prioritizing pre-fumigation cleaning, you protect your products, ensure compliance, and maximize the effectiveness of the fumigation process. Remember: Clean first, sanitize second!
At ACM, we understand that cleanroom supplies and equipment are just as critical as cleaning techniques. With over 30 years of experience, we meticulously select supplies and tools that meet the unique and specific cleaning demands of your cleanroom while ensuring the elimination of recontamination risks.
Specialized Supplies and Equipment
-
Cleanroom Detergents:
- All detergents are ULPA-filtered to 0.1 microns, ensuring no particles are introduced during the cleaning process.
- Solutions are tailored to meet your cleanroom’s requirements, from general cleaning to specialized needs like particle removal or surface decontamination.
-
Disinfectants:
- Selected based on:
- Bio-contamination risks: Addressing bacteria, fungi, molds, and viruses.
- Rotational protocols: Preventing resistance by alternating disinfectants.
- Commodity compatibility: Ensuring safe application on materials and surfaces in your cleanroom.
- Disinfectants are rigorously tested for effectiveness and compliance with industry standards.
- Selected based on:
-
Vacuum Cleaners:
- Equipped with exhaust systems filtered to 0.1 microns, preventing particles from being reintroduced into the cleanroom environment.
- Designed for cleanroom use, ensuring maximum efficiency without generating secondary contamination.
-
Cleanroom-Approved Wipes:
- Selected for surface compatibility, ensuring no damage to sensitive equipment or materials.
- Designed to reduce particle shedding and provide streak-free cleaning.
ACM’s Customized Solutions
ACM associates go beyond simply providing supplies—we work with you to identify and implement the most appropriate tools and products for your cleanroom environment:
- Tailored Product Selection: We assess your facility’s needs, including the materials, processes, and contamination risks, to recommend the best supplies.
- Compatibility Assurance: Products are carefully chosen to avoid damaging surfaces, disrupting production, or causing recontamination.
- Expert Guidance: Our team advises on proper usage, storage, and disposal of cleaning supplies to maintain optimal performance and compliance.
Why Supplies and Equipment Matter
Cleanroom supplies and equipment are integral to maintaining a contamination-free environment. With ACM’s expertise, you can trust that every product and tool used in your cleanroom will:
- Meet or exceed ISO and GMP standards.
- Maximize cleaning effectiveness while minimizing recontamination risks.
- Support your specific cleaning protocols and regulatory compliance needs.
By combining the right supplies with ACM’s proven methodologies, your cleanroom is maintained at the highest level of cleanliness and operational integrity.
At ACM, we understand that every cleanroom is unique, with its own challenges and requirements. Whether your primary concern is controlling viable particles, optimizing yield, or maintaining compliance, ACM associates develop tailored programs to ensure your cleanroom operates at peak performance.
Our Commitment: Guaranteed Effortless Compliance
When you partner with ACM, you gain access to a team of certified professionals dedicated to delivering:
-
Unparalleled Expertise:
- With over 30 years of experience, ACM is a trusted leader in cleanroom cleaning, certification, and compliance.
- Our team applies advanced methodologies and the latest technologies to address even the most challenging contamination issues.
-
Customized Cleaning Programs:
- We design programs tailored to your industry, facility, and product requirements.
- From viable particle control to maximizing production yields, our solutions are crafted to meet your specific goals.
-
Measurable Results:
- ACM’s cleaning methods consistently reduce particles by three to ten times, providing a cleaner and safer environment for your operations.
- Improved cleanliness directly supports better yields, fewer defects, and increased profitability.
-
Effortless Compliance and Reporting:
- Our team handles all the paperwork, documentation, and regulatory requirements for you.
- We create detailed, audit-ready reports to ensure compliance with ISO, GMP, FDA, and other regulatory bodies.
What We Offer:
-
Peak Cleanroom Performance:
- ACM helps you maintain an optimal environment to protect your products, processes, and reputation.
-
Productivity and Efficiency Gains:
- Cleaner environments lead to higher production yields and fewer operational interruptions.
-
Hassle-Free Management:
- Let ACM handle the technical and administrative tasks so you can focus on your core operations.
Take the Next Step
Experience the ACM difference for yourself. Contact us today and let our team of experts:
- Keep your cleanroom operating at its best.
- Increase your productivity and profitability.
- Simplify compliance, reporting, and documentation.
From three to ten times cleaner, effortlessly—choose ACM!

