GMP & Cleanroom Cleaning
Maintaining a cleanroom environment that adheres to Good Manufacturing Practices (GMP) is essential for industries such as pharmaceuticals, biotechnology, and medical device manufacturing. ACM offers comprehensive GMP cleanroom cleaning services designed to ensure compliance with stringent regulatory standards and enhance product quality.
GMP & Cleanroom Cleaning
Maintaining a cleanroom environment that adheres to Good Manufacturing Practices (GMP) is essential for industries such as pharmaceuticals, biotechnology, and medical device manufacturing. ACM offers comprehensive GMP cleanroom cleaning services designed to ensure compliance with stringent regulatory standards and enhance product quality.

Clean-Build Cleaning and Construction Supercleans
Clean-Build is a process of starting to clean, building, and staying clean. There are five levels of clean construction protocols. ACM is proud to have the best clean-build construction cleaning methods.
Protoclean cleaning starts at Protocol Level 1 and ends with the completion of Certification at Level 5. Supercleans are performed before transitioning to each Protocol Level.
The cleaning may include any or all of the following:
If you need to construct a cleanroom, we will be glad to communicate the necessary protocols for cleaning and construction, including Protocol training, temporary gowning, material entry rooms, monitoring the Clean-build process, cleanroom supplies, and garments for each of the five levels.
ISO 14644-4 defines the Clean-build protocols and specifications for application when designing a cleanroom and construction methodology.
Post-Construction GMP Validation Cleaning
Validation cleaning covers all aspects of the GMP cleaning procedures to validate predictable results as documented in the SOPs and used to set up alert and action limits. Further changes to the SOPs past successful validation require re-validation. Validation is usually confirmed by the statistical process of the environmental monitoring results. Validation cleaning may include changes in disinfectants, sporicides, cleaning materials, and wipes.
Non-GMP Cleaning (Janitorial)
ACM‘s sophisticated systems used for cleanroom cleaning and testing operations are simply converted to office and industrial janitorial services for our already satisfied cleanroom customers.
Some of the janitorial services we offer using “green” sustainability cleaning and products that are safe for the environment and people are:
For more information on our Janitorial cleaning service, visit our page HERE.

Ancillary Benefits of ACM Services
Our company develops a program tailored to your facility, product, and process needs. Our cleaning programs deliver these additional cleanroom services:

SOP Development and Maintenance
SOPs (Standard Operating Procedures) may be developed for any of our services, allowing for continued compliance with the best industry practices. SOPs are continuously maintained and edited for improvements and industry changes.
Personnel and Cleaning Workflow Guidance
Training, training, and more training. Good habits are best maintained with regularly scheduled certified training according to our ISO 9001:2015 systems, customer SOPs, and IEST cleanroom Recommended Practices for cleaning methodologies.
Periodic Facilities and Personnel Inspection
We proactively conduct inspections to ensure your facility and team are always prepared for any formal evaluations. Achieving high scores not only boosts morale but also contributes to increased profitability, keeping management satisfied.
Cleanroom Policing
To ensure unbiased oversight, ACM offers cleanroom monitoring services. By having an external team perform this role, we help maintain the integrity and safety of your cleanroom environment and are available on any schedule that suits your operations.
ACM’s GMP Cleanroom Cleaning
ACM’s™ ISO 9001:2015 Certified GMP cleanroom cleaning reduces non-viable (dust, metals, fibers, salts, bases, smoke, etc.) and viable (bioburden) particles. ACM technicians use non-linting and microfiber materials with distinct properties that match the surfaces in your facility. The type of contamination, whether viable or non-viable, is targeted. Microcleaning is a key factor in product compliance and yield improvements, both contributing to higher customer profitability.
See: ACM’s Microclean vs. Janitorial Cleaning
ACM’s™ cleanroom cleaning knowledge is at the expert level, and we have over 20 online classes for our Cleaning Technicians. The training consists of Good Documentation Practices (GDPs), cleaning methodology, cleanroom technology, safety, microbiology, and aseptic techniques. Once classroom instruction is completed, the technician then studies the customer’s SOPs before starting site-specific on-the-job training.
ACM’s training methodology of starting from the cleanest end of the room, farthest from the exit, cleaning top down, ceilings, walls and lastly floors has proven to decrease deviations by over 200 times or improved yields up to 99% at most large companies.
Please see the bottom of the page for details on ACM’s™ cleaning methodology for Computer Rooms.
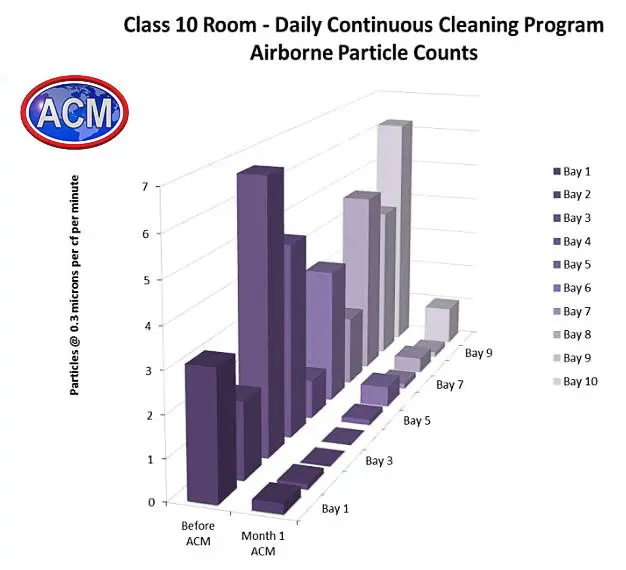
GMP Cleanroom Cleaning
GMP (Good Manufacturing Practices) and cGMP (Current Good Manufacturing Practices) set the standard for manufacturing food, drugs, medical devices, and cosmetics in sanitary conditions. The FDA’s CFR Title 21 mandates that facilities involved in the manufacturing or storage of these products maintain a clean and sanitized environment to ensure consumer safety.
Key Cleaning Processes
Cleanroom Cleaning Specifics
While the methodology for cleanroom cleaning mirrors that of GMP cleaning, it incorporates the use of specific cleaning agents, such as cleanroom detergents (e.g., Novaclean) or DI water/isopropyl alcohol blends (e.g., Protohol products), instead of traditional disinfectants. The procedure involves starting from the area furthest from the exit and cleaning in a top-down, side-to-side manner to effectively remove particulates. Additional steps, like vacuuming with HEPA-filtered Nilfisk vacuums or using cloths to capture particles as small as 0.25 microns, may precede the liquid cleaning phase, enhancing the overall efficacy of cleanroom maintenance.
GMP Sporicide Cleaning
A sporicide is required for the effective elimination of spore-forming microorganisms. This process is usually performed during the initial validation cleaning methods and periodically thereafter, based on the validation outcome and documented SOPs.
GMP Cleaning SOP Documentation
ACM creates, documents, and delivers GMP SOPs (Good Manufacturing Practices, Standard Operating Procedures) based on customer requirements. ACM provides continuous improvement in the SOP documentation for both ACM SOPs and the customer's SOPs. Facilities and ACM'sTM technicians are trained and certified on the SOPs and on GMPs for Documentation. For effortless compliance, our SOPs’ are written by area experts to comply with the most current international regulatory agencies' requirements.
Supercleans
Supercleaners start at the ceiling or highest point and continue down the walls to the floor level. Superclean cleaning also starts in the area farthest from the exit door and works out towards the least clean area, the entry/exit. The number and frequency of Supercleans vary depending on the requirements of the project.
Supercleans may include any or all of these cleanings: Top-down Cleaning, Air Handler Unit (AHU) Cleaning, Raised Floor Cleaning, Plenum Area Cleaning, High-Bay Cleaning, Computer Room and Data Center cleaning.
Computer Rooms and Data Centers
The computer room needs to be cleaned to maintain the computer equipment's integrity. ACM guarantees to remove dirt, dust, and other contaminants from your computer rooms, leaving them 3–10 times cleaner.
ACM’s™ certified technicians clean under the raised floors, the tops of floors, the room and equipment interiors, and exteriors. For example, in a cleanroom, cleaning is performed from the area farthest from the exit but cleaned from the bottom up. Wipes, mops, and cleaning solutions are selected for equipment and surface compatibility. Any particles not removed by the Nilfisk HEPA-filtered vacuums are removed by surface surfactants down to 0.5 microns.
The raised floor plenum requires routine cleaning. Even with the clean air supply from the plenum, gravity alone causes contaminates to settle under the floor. It doesn't take long for the particles to accumulate, settle out, and be deposited in your equipment by the air supply under pressure designed to cool your equipment.
ACM's cleaning processes are proven to remove particles and maintain a clean environment for your equipment and personnel.
Call ACM for Guaranteed Effortless Compliance
ACM ensures your operations excel by minimizing cleaning-related deviations, enhancing product process compliance, and boosting yields. The cost of poor quality (COPQ) could represent a significant loss—up to 25-50% of a company’s total revenue. Moreover, deviations could cost upwards of $20,000 each, not to mention the potential for product delays and FDA violations.
Our certified technicians oversee your cleaning program, allowing your team to concentrate on what matters most: the production process. For detailed information on ACM’s GMP and cleanroom cleaning services, explore our FAQ section or get in touch today. Discover how ACM provides stress-free compliance solutions tailored to your facility’s needs.


